Unlock Your Potential with Advanced Clinical Research Institute
Advancing Discovery, Transforming Lives: Your Journey Begins Here
Enroll in our premier clinical research course today!
Expert Faculty
Placement Assistance
Industry Relevant Skills
Learning Experience
Prospective Employers for Your Professional Journey
Empowering Your Journey: Connecting You with Potential Employers for a Bright Career Ahead!












Introduction to Cutting-Edge Clinical Research Courses:
Introduction to the cutting-edge methodologies and technologies covered in the courses.
Immerse yourself in a diverse selection of Clinical Research courses designed to provide you with essential skills for the ever-changing healthcare landscape. Stay ahead in this rapidly advancing field and excel with our innovative programs.

Duration- 6 Months
PG – Diploma In Advanced Clinical Research And Pharmacovigilance
AIICR Course Curriculum
PG Diploma In Advanced Clinical Research And Pharmacovigilance
- Introduction To Clinical Research
- Drug Discovery & New Drug Development
- Basic Terminology Used in Clinical Research
- Investigational New Drug
- Clinical Trial and Design
- Guidelines For Clinical Trials (ICH-GCP)
- Ethics In Clinical Research
- Regulations In Clinical Research
- Quality In Clinical Trials
- Roles And Responsibilities in Clinical Research
- Introduction To Clinical Research
- Drug Discovery & New Drug Development
- Basic Terminology Used in Clinical Research
- Investigational New Drug
- Clinical Trial and Design
- Guidelines For Clinical Trials (ICH-GCP)
- Ethics In Clinical Research
- Regulations In Clinical Research
- Quality In Clinical Trials
- Roles And Responsibilities in Clinical Research
- Fundamentals of Biostatistics
- Statistical Tests
- Survival Analysis
- Introduction to Pharmacovigilance
- History of Pharmacovigilance
- Development of Pharmacovigilance
- Importance of safety monitoring of medicine
- WHO International Drug Monitoring Programme
- Pharmacovigilance Program Of India (PvPI)
- Introduction to Adverse Drug Reaction
- Detection and Reporting of ADR
- Causality assessment
- Management of Adverse Drug Reactions
- Drug And Disease Classification
- International classification of diseases
- Daily defined doses
- International nonproprietary names for drugs
- Drug Dictionaries and Coding in Pharmacovigilance
- MedDRA and standardized MedDRA queries
- Standardized MedDRA Queries
- WHO Drug Dictionary
- Eudravigilance medicinal product dictionary
- Information Resources in Pharmacovigilance
- Specialized resources for adverse drug reactions
- Basic steps in setting up a pharmacovigilance center
- Contract Research Organization (CRO’s)
- Establishing a national programme:
- Vaccine Safety Surveillance
- Pharmacovigilance methods
- Communication in drug safety crisis management
- ICH Guidelines for Pharmacovigilance
- Individual Case Safety Reports
- Periodic Safety Update Reports
- Post Approval Expedited Reporting
- Centre for International Organizations of Medical Sciences (CIOMS)
- Drug And Cosmetic Act
- ADR Reporting Procedure of India VS ADR Reporting procedure of USA
- Career guide
- Introduction to medical writing
- Scientific Writing
- Pre-clinical or Non-Clinical writing
- Medical Writing for Clinical Trial
- Medical Writing in the post-marketing phase
- Guidelines to publish clinical data
- Medico-marketing writing
- Medical & Scientific Style guides
- Data Citation & referencing
- Plagiarism & Copyright
- Introduction to SAS
- Case Study Using SAS
- Introduction to Regulatory Affairs
- Drug regulatory and accrediting agencies of the worlds
- Regulatory Document
- Non-Clinical Study Reports (M4S)
- Clinical Trials (E3)
- Provisions related to the Import of Drugs
- DMF- preparation, submission, responding to regulatory deficiencies
- Importance of GMP Compliance
- Orange book
- Biologics License Application (BLA) and Purple Book
- QA/QC Documentation
- Hatch Waxman Act- 180-day exclusivity
- Patent- Fundamental Concept
- HIPPA Act
Our expert consultants will confidently guide you through clinical research with personalized assistance.
Why Choose AIICR?
The Advanced International Institute of Clinical Research is a progressive and premier clinical research institute that has rapidly gained a good reputation for excellence through its innovative approach to clinical research and medical coding.

100% placement support

Online & offline classes

Life time app access

Affordable fee

Monthly Instalment

Expert faculty

Industry relevant curriculum

Clearing doubt Anytime
Enroll and get a free live demo
Recent Placements

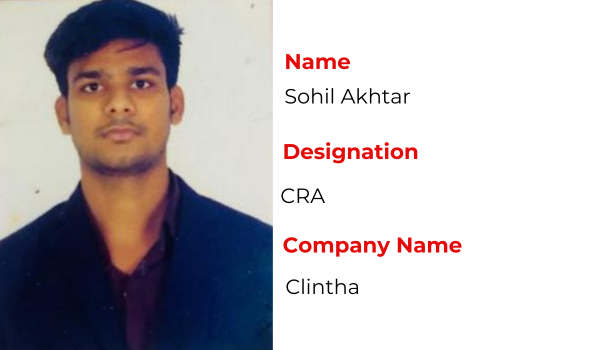
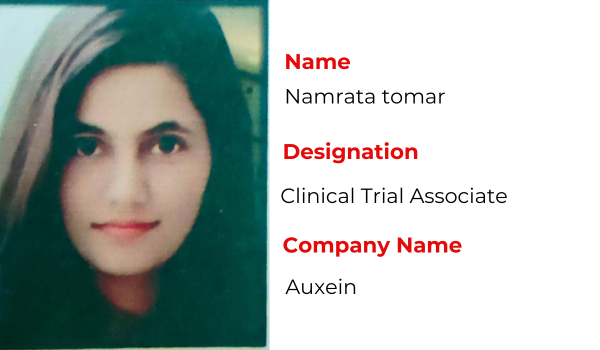
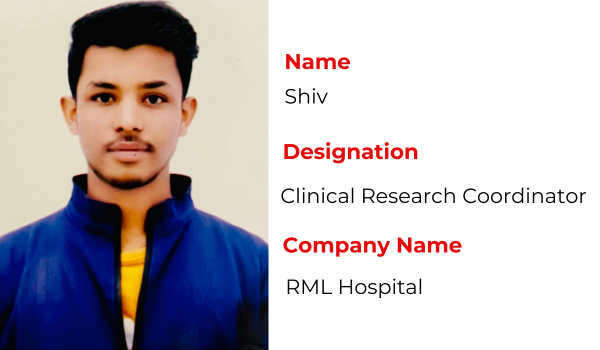



Hear the Success Stories
Where Aspiring Minds Meet Exceptional Opportunities
I had an incredible experience at AIICR. The advanced diploma program was exactly what I needed to kickstart my career in clinical research. The curriculum was comprehensive and covered everything from the basics to advanced concepts. The faculty was knowledgeable and always willing to help. Thanks to the excellent placement department, I secured a job at a top pharmaceutical company right after graduation. I highly recommend AIICR to anyone looking to excel in this field.

Ismeet Singh
AIICR provided me with the perfect blend of theoretical knowledge and practical skills. The courses were well-structured and the instructors were experts in their fields. They made even the most complex topics easy to understand. The admin staff was supportive and always available to assist with any issues. The placement department did a fantastic job in helping me prepare for interviews and ultimately land a great job. I am grateful for the education and support I received at AIICR.

Visakha
Enrolling in the advanced diploma program at AIICR was one of the best decisions I ever made. The curriculum is rigorous and up-to-date with industry standards, ensuring that students are well-prepared for real-world challenges. The faculty members are not only knowledgeable but also genuinely invested in their students’ success. The placement team was incredibly supportive and helped me secure a position in a leading clinical research organization. AIICR has truly set me on the path to a successful career.

Uttam Jain
AIICR’s advanced diploma program in clinical research exceeded all my expectations. The curriculum was thorough and relevant, covering all essential aspects of clinical research. The faculty was outstanding, always ready to provide guidance and support. The administrative staff ensured a smooth and hassle-free experience from enrollment to graduation. The placement department’s efforts were instrumental in helping me land a great job. I am incredibly thankful for my time at AIICR and the opportunities it has opened up for me.

Sandeeep Saini
I just want to express that enrolling in AIICR was the best decision I ever made. The guidance provided by AIICR’s faculty has been immensely beneficial to me. Moreover, I owe a great deal to the placement department for their continuous support until I secured my job. They stayed connected with me until I landed the right opportunity. I am sincerely grateful to AIICR for their invaluable support. To all the students who are considering their career paths, especially in clinical research, I highly recommend joining AIICR. Trust me, it will be a decision you won’t regret. Thank you so much, AIICR, for everything. To all the aspiring students out there, if you aspire to build a career in clinical research, I wholeheartedly recommend AIICR.

Sumit Rohilla
Studying at AIICR was a rewarding experience. The advanced diploma program was well-designed and provided a comprehensive understanding of clinical research. The teachers were passionate and dedicated, making learning an enjoyable experience. The admin staff was friendly and always willing to help with any queries. The placement department played a crucial role in helping me secure my first job in the industry. I am grateful for the education and support I received at AIICR and would highly recommend this institute to prospective students.

Daksh Jain
My time at AIICR was transformative. The advanced diploma program provided a solid foundation in clinical research and equipped me with the skills necessary to excel in the industry. The instructors were exceptional, offering valuable insights and practical knowledge. The admin staff was efficient and supportive throughout my studies. The placement department went above and beyond to help me secure a job in a prestigious research organization. I am proud to be an AIICR alumna and highly recommend this institute.

Navjeet Malik
AIICR has been a pivotal step in my professional journey. The advanced diploma program offered a robust curriculum that was both comprehensive and up-to-date with current industry practices. The faculty members were exceptionally knowledgeable and provided invaluable insights and mentorship. The administrative staff ensured a smooth academic experience, always addressing any concerns promptly. The placement department’s guidance and resources were instrumental in helping me secure a position with a renowned clinical research organization soon after graduation. I highly recommend AIICR for anyone serious about a career in clinical research.

Uncover The Brilliance Within And Achieve Recognition Through These Remarkable Certifications
Unlock Career Success with a Resume/CV that Propels You Towards Achievement



Enroll and get a free live demo
Copyright 2023, AIICR (Advanced International Institute of Clinical Research.)
